名古屋大学 大学院理学研究科 物質理学専攻(化学系) 無機化学研究室
We design and prepare new inorganic catalysts by using metal complexes and metal nanostructures for selective catalysis.
Research 1
Development of a new preparation method for surface-attached metal nanocluster catalysts
with regulated size and composition distribution
Metal nanocluster catalysts in which several numbers of metal atoms are assembled exhibit unique catalytic performances that are not seen in monometallic metal complexes due to their metal-metal bonding interactions. The attachment of metal nanocluster catalysts on an appropriate surface makes them more stable and recyclable during catalytic reaction.
The preparation methods of metal nanoparticles up to date yield metal nanoparticles of wide size and composition distribution. Therefore, it is difficult to prepare, or ‘synthesize’, the metal nanoparticle of special size and composition that has special catalytic activity. It is also difficult to clarify the relationship between catalytically active structures and catalytic reactions, that is, the mechanism of catalytic reaction at a molecular level. Moreover, simple supported metal nanoparticles undergo sintering during the catalytic reaction, and it is difficult to maintain their catalytic activity. So far, no methods for the preparation of metal nanoparticles of regulated size and composition distribution using various combinations of metal atoms directly on the surface.
We aim to develop a new preparation method for metal nanocluster catalysts with regulated size and composition distribution directly on the surface. Through this method, we will be able to prepare any types of metal nanocluster catalysts with regulated size and composition distribution. It will be possible to investigate new catalytic reactions that are inherent to the new metal nanoparticles. Since the size and composition distribution are united, it will be possible to understand the relationship between catalytically active structures and catalytic reactions in a similar manner to that of metal complexes.
We investigated the way of preparing highly-dispersed Ru3 cluster on SiO2 surface and the further structural transformation of the supported Ru3 cluster to a dispersed Ru nanocluster on the SiO2 surface, and we succeeded in preparing dispersed Ru nanoclusters, whose average diameters were 1.3 ± 0.3 nm through structural transformation methods (Figure 1). The supported Ru nanoclusters were found to be active for the selective oxidation of a variety of alcohols to their corresponding aldehydes using O2. We are now investigating the methods of assembling a desired number of metal complexes on SiO2 surface. The regulation of the number of metal complexes assembled on surfaces is the key point to prepare metal nanoclusters of regulated size and contribution distribution. We are also trying to directly convert the supported metal complex assemblies to metal nanoclusters through surface isolation methods and structural transformation methods.
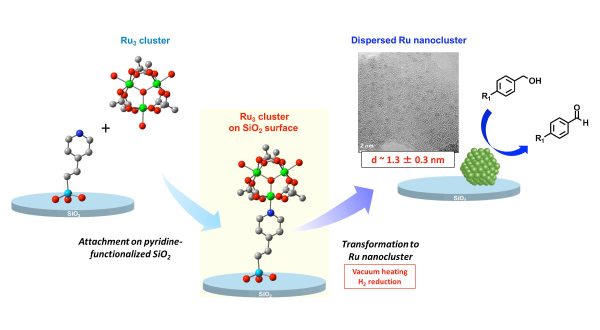
Figure 1 Schematics of the grafting of Ru3 trinuclear cluster on the pyridine-functionalized SiO2 surface through coordination interaction and the transformation to Ru nanoclusters with high dispersion for catalytic alcohol oxidation.
References
1. S. Muratsugu, M. H. Lim, T. Itoh, W. Thumrongpatanaraks, M. Kondo, S. Masaoka, T. S. A. Hor, and M. Tada, Dalton Trans., 42, 12611-12619 (2013). (selected as an inside front cover).
Research 2
Design and preparation of molecularly-imprinted metal complex catalysts
for selective catalytic reaction
A number of useful chemical products have been produced by heterogeneous catalysts and the development of new heterogeneous catalysts with high activity, selectivity, and stability is one of the challenging issues in many fields in chemistry. Natural enzymes are sophisticated catalytic systems capable of molecular recognition using a special interaction with particular reactants and binding sites (e.g. hydrogen bonding), and they are role models of catalysts that are selective for particular reactants. Enzymes use specialized three-dimensional active sites to control and carry out catalytic reactions. The design of such active sites in non-enzymatic systems remains a challenging endeavor for developing new catalysts.
We develop molecularly imprinted metal complex catalysts on oxide surfaces inspired by natural enzymes. “Molecular Imprinting” is a method for creating, in a polymer matrix, an imprinted cavity that has a shape matched to a template molecule. We have been designed and prepared a new molecular imprinting method of which one of ligands coordinated to a supported metal complex on an oxide surface is used as “a template” (Figure 1). Supported metal complexes, which are superior to homogeneous metal complexes from the point of view of site isolation, high stability, and new active sites, is used for the preparation of molecularly-imprinted metal complex catalysts. A special ligand, called a template ligand, is coordinated to the metal center of the supported metal complex. Then, thin matrix overlayers made of organic/inorganic polymers are stacked around the supported metal complex. Finally the template ligand is selectively removed from the metal complex. In this way, an imprinted cavity with a similar shape to the template for shape selective catalytic reaction is constructed in the close proximity of an unsaturated active metal center. The matrix overlayers serves as a protector of the active metal species as well as to keep the shape of the imprinted cavity.
We have designed and prepared a molecularly-imprinted Ru complex catalyst for regioselective epoxidation of (R)-(+)-limonene, which is able to undergo selective epoxidation of the terminal C=C bond of (R)-(+)-limonene (Figure 2). We have also developed a molecularly-imprinted Ru complex catalyst with a molecular binding site on the wall of a molecularly imprinted cavity on an oxide surface, and investigated the effect of the binding site to selective asymmetric transfer hydrogenation catalysis of o-fluorobenzophenone. We are now developing new molecularly-imprinted metal complex catalysts for regulation a selective transformation of useful compounds such as sugar molecules. The design of molecularly imprinted metal complex catalysts on oxide surfaces will greatly contribute to the preparation of artificial enzymatic surfaces.
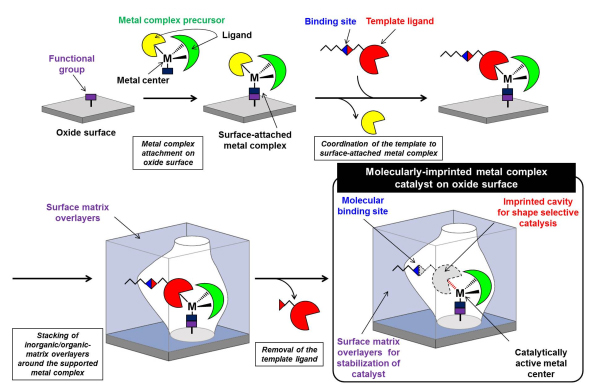
Figure 1 A preparation scheme for a molecularly-imprinted metal complex catalyst on an oxide surface.
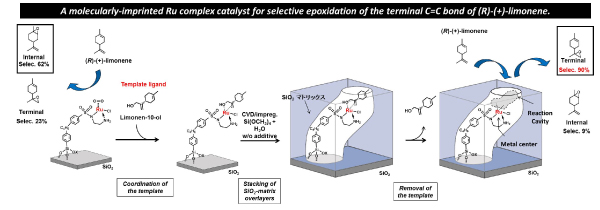
Figure 2 A preparation steps for the molecularly-imprinted Ru complex catalyst for selective epoxidation of the terminal C=C bond of (R)-(+)-limonene.
References
1. S. Muratsugu,and M. Tada, Acc. Chem. Res. 46, 300-311 (2013,).
2. Y. Yang, Z. Weng, S. Muratsugu, N. Ishiguro, S. Ohkoshi, and M. Tada, Chem. $2013Eur. J. 18, 1142-1153 (2012).
3. Z. Weng, S.Muratsugu, N.Ishiguro, S.Ohkoshi, and M. Tada, Dalton Trans. 40, 2338-2347 (2011).
Research 3
Transition metal cluster molecules at sub-nanometer scale
Metal particles in nano-size are termed as metal nanoparticles. Their properties, such as the color, magnetism, and reactivity, are known to vary significantly by their size and structure. From the viewpoint of potential catalytic applications, particle diameters around or slightly less than 1nm (sub-nanometer-sized metal particles) are important, while difficulties remain in the size- and structure-selective synthesis. Our particular interest resides in the size- and structure-selective synthesis of sub-nanometer-sized metal particles in the molecular level, which are soluble and isolable. In other words, our target compounds in this research topic are "metal cluster molecules at sub-nanometer scale". Of course we are not the only players in this research field, and some interesting works have been done with noble metals particularly gold and platinum. The clusters with more abundant iron-group elements (Fe, Co, Ni) are main focus of our study.
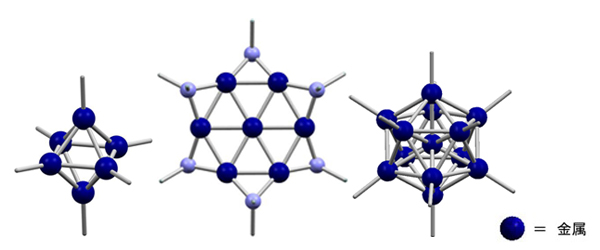
Someone may compare the size- and structure-selective synthesis of subnano-scaled metal clusters to the synthesis of macromolecules in a specific chain length. An apparent difficulty here is how to avoid (or minimize) the distribution of size and structure of the products. In order to overcome the difficulty, we are trying to develop our own synthetic methods. As shown in the figure, one of our methods enabled us to produce some beautiful metal-cluster molecules.
Research 4
Microstructure Analysis of an Individual Catalyst Particle using X-ray Nanobeam
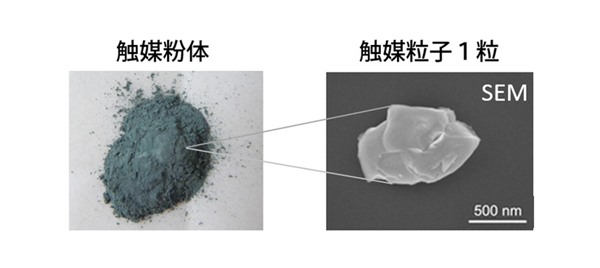
Heterogeneous solid catalysts have been widely used for various catalytic syntheses. In general, solid catalyst particles are assemblies of individual powder particles with nano-micron meter sizes. These particles with different sizes, shapes, and local structures are expected to exhibit different catalytic performances depending on their local microstructures. However, the local structures of individual catalyst particles cannot be revealed by conventional analytical techniques and it is still difficult to understand the microstructures of individual catalyst particles and their catalytic performances.
We have developed nano-XAFS (X-ray Absorption Fine Structure) technique at SPring-8, whose X-ray nanobeams focused by KB mirrors are used. X-ray nanobeams from synchrotron are irradiated on individual catalyst particles dispersed on a membrane substrate, and fluorescent X-rays from the individual particles are detected to find the chemical information and local structures of the individual catalyst particles. Recently, we have succeeded in visualizing local structures of supported Ni catalyst on a single particle of Ni/Ce2Zr2Ox for CH4 steam reforming and the heterogeneous distribution of Ce oxidation states inside a single catalyst particle of Pt/Ce2Zr2Ox for automobile exhausting.
Research 5
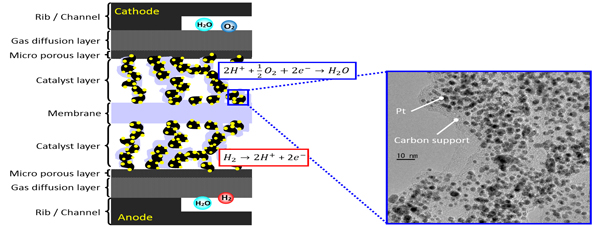
In situ 3D Structural Analysis of Catalyst Layers in Polymer Electrolyte Fuel Cells
Polymer Electrolyte Fuel Cells (PEFCs) are one of the promising energy sources and have been applied to various systems such as automobiles. Pt nanoparticles dispersed on carbon supports are generally used as electrocatalysts. At anode, hydrogen converts to proton and electron on an anode catalyst and the formed proton is transferred to the cathode surface. At cathode, a Pt cathode catalyst reacts with proton and oxygen to form water.
It is well known that the degradation of a cathode catalyst significantly proceeds during PEFC operation. There are many approaches by electrochemical analysis techniques to elucidate the mechanism of the degradation of a cathode catalyst, however it is still difficult to understand the degradation phenomena of the cathode electrocatalyst under PEFC working conditions.
We have developed in situ time-resolved/space-resolved XAFS techniques at SPring-8 Synchrotron facility, and applied them to Pt and Pt-alloy electrocatalysts in PEFC. In situ time-resolved XAFS provides structural kinetics and reaction mechanism of cathode electrocatalysts under PEFC operating conditions, and we elucidated the kinetic parameters of redox reactions of the Pt cathode catalyst for voltage-operating processes. The roles of second metals (e.g. Co) in the cathode alloy catalysts were also investigated by the in situ time-resolved XAFS analysis. Laminography-XAFS is developed for the visualization of three-dimensional morphology and chemical state distribution of catalyst layers in membrane electrode assembly for PEFCs.